Focus: protein enzyme-coupled receptors and proteolysis
6 main classes of enzyme-coupled receptors:
1) Receptor tyrosine kinases
- transmembrane proteins
- one class of these bind secreted proteins (proteins floating around in extracellular space) and another class bind cell surface proteins
- all have tyrosine kinase domains on the inside of the cell
- many are involved in the growth and proliferation of cells
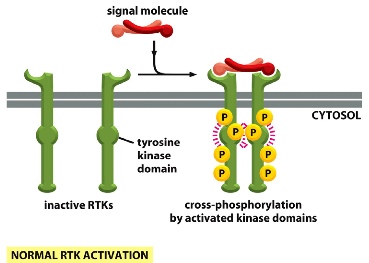
Ligand on the outside of the cell binds two receptors and cause them to dimerize. The final product then is able to phosphorylate each other: auto-phosphorylation —> signals other phosphorylation happening on the tail.
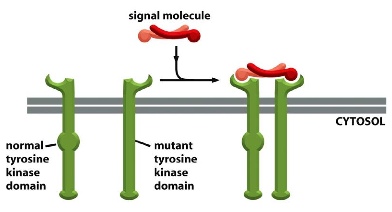
What happens if one of the two receptor is mutated? —> depending on the ratio of normal complex vs. mutant complex.
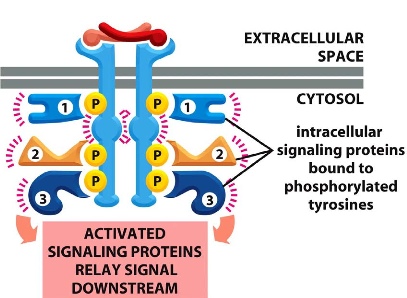
phosphorylated sites act like binding sites for the intracellular signally proteins (contain domains SH2 or PTB domain that bind to phosphotyrosine and neighbouring sequences)
e.g. PDGF receptors phosphorylate themselves and then recruit other proteins that contain additional domains/ act as adaptors to recruit proteins with no SH2 domains.
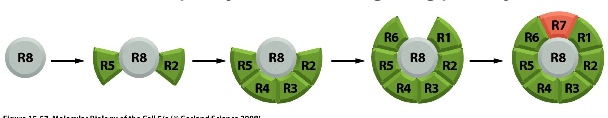
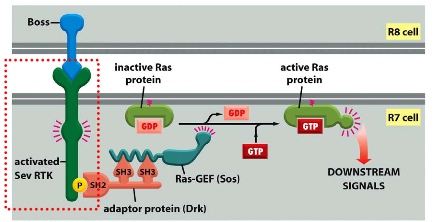
Pathway is discovered through the study of Drosophila
sequential differentiation —> 8 photoreceptors —> R7 is needed to detect UV light (screening them to see if a mutant that fails to detect UV exists)
The mutant identified is called Sevenless: normal sevenless protein is a receptor tyrosine kinase expressed in R7 cells.
If you mutate Sevenless, you will not have R7.
BOSS = bride of sevenless is found on the R8 cell and is a ligand which binds to the sevenless receptor
SOS = son of sevenless (GEF)
BOSS activates Sev RTK —> allowing adaptor protein Drk to bind which activats Ras-GEF(SOS) —> exchanges GDP to GTP on Ras protein and activates it.
Ras is a superfamily with members that mainly function to be involved in cell proliferation and differentiation.
- monomeric GTPase
- activated by Ras-GEFs and inactivated by Ras-GAPs
- hyperactive mutant forms of Ras contributes to tumour (uncontrollable growth)
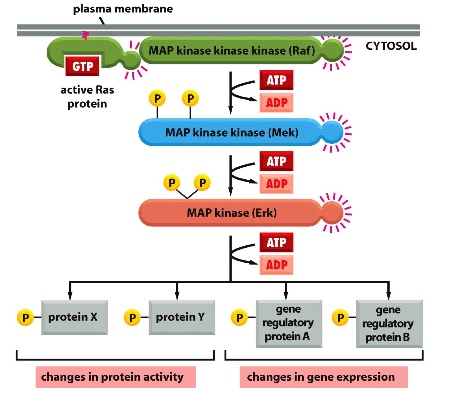
Ras activates MAP kinase module (mitogen-activated protein kinase module) to chafe protein activity and gene expression —>cell growth and division
involving a relay of phosphorylation of the different kinases
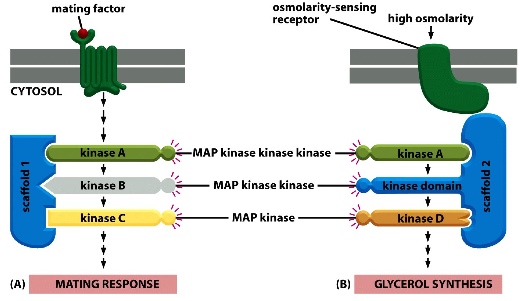
There can be many parallel pathways operating at the same time. The scaffold protein forces different kinases to form combos that relay signals down to have different responses. If the combo is changed, experiments have shown that an altered product is made.
2) Tyrosine-kinase-associated receptors: where receptors interact with a cytoplamic tyrosine kinase non-covalently.
3) Receptor serine/threonine kinases
- largest class of cell surface receptors in plants, also function in animals
- 6 major families
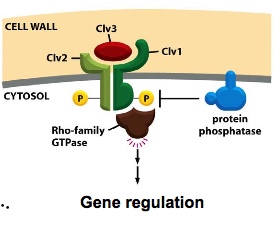
e.g. undifferentiated cells in plants where plants grow stems, leaves and flowers:
Clv 1 is a serine/threonine kinase. —> creates docking site for Rho-family GTPase
Clv 3 is ligand.
4) Histidine - kinase -associated receptors
used b/t bacteria, yeasts and plants only
Flagella moves in a single direction normally, but when it encounters repellent, the flagella moves in the opposite direction and tumbles as a result. Here, repellent binds to chemotaxis receptor —> auto-phosphorylation of CheA, a histidine kinase —> CheA transfers the PO4 to an aspartic acid on CheY.
Use of proteolysis in signalling
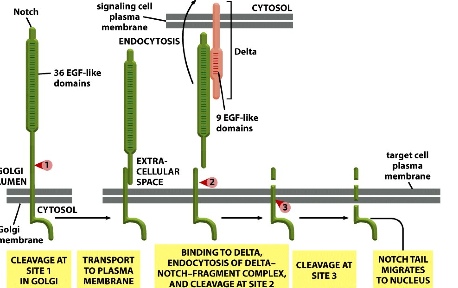
Notch signalling
at the beginning of differentiation, there is low level of signalling for all the cells, but when a few cells gain advantage, they have stronger signals and they prevent other cells from getting the signals.
Cell with the active Delta becomes specialized.
Cell with the inactive Notch becomes specialized.
Active Notch sends out the inhibition signal.
1. Covalent breakage of Notch receptor
2. Fragment reassociates with itself in Golgi lumen using non-covalent interaction (weaker)
3. Delta binds to cleaved fragment- —> binds to Notch and pulls it off its anchor (it is proposed that upon binding, Delta is endocytosed which drives the separation of the fragments)
4. the left-over anchor is cleaved at 2 sites
5. tail of Notch enters nucleus, where it recruits other proteins to form a complex and bind to DNA
6. transcription of Notch target genes
Sonic hedgehog signal (Shh)
Shh spreads from its source as a ligand, and the gradient controls the formation of distinct digits.
<cytoplasm>
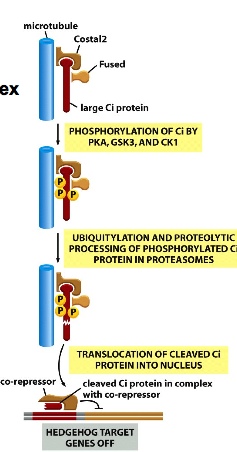
Without Shh, there is inactive machinery. Large Ci proteins binding to Costal2 and attached to microtubule, and is kept in the cytosol of the cell —> inside cilia. In addition, the microtubule-associating complex promotes the proteolysis(ubiqination and proteosome activity) of Ci and uses the cleaved Ci protein as a repressor which then binds to DNA to turn Shh target genes off.
<plasma membrane>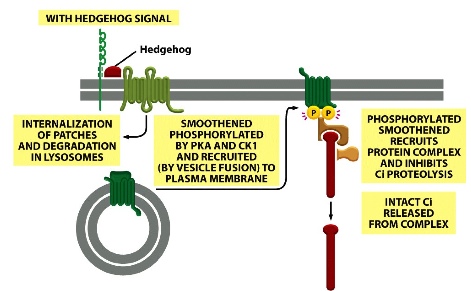
Meanwhile, the Smoothened protein is being kept inactive by the Patched protein. Smoothened stays in intracellular vesicles until Shh signal comes in and releases Patched. Now Smottened can by phosphorylated and recruits p
rotein complex that contains Ci —> intact Ci can enter nucleus —> binds to DNA and activates transcription.